Calcium and phosphate levels in adult patients receiving VELTASSA during clinical studies
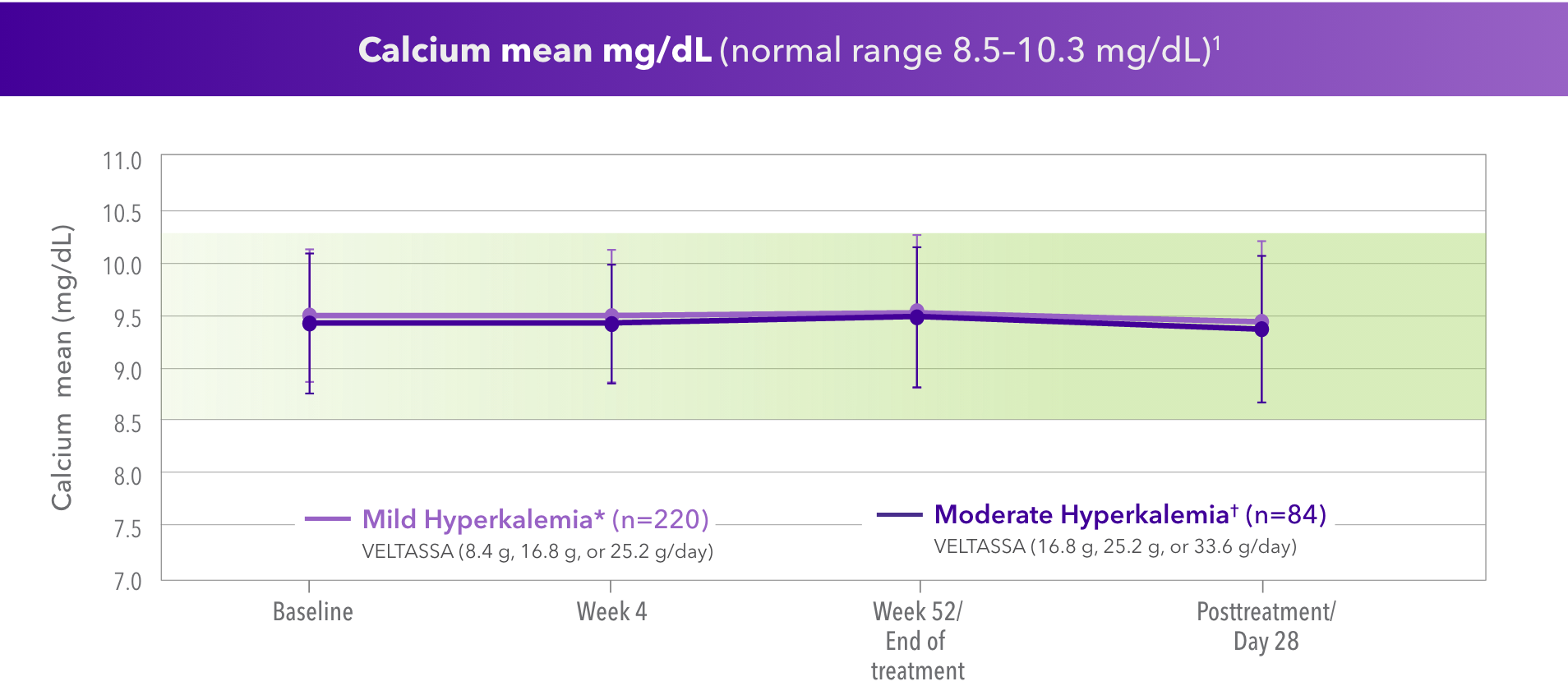
For Calcium
- No clinically relevant changes in serum calcium after 52 weeks when dosed up to 33.6 g/day‡
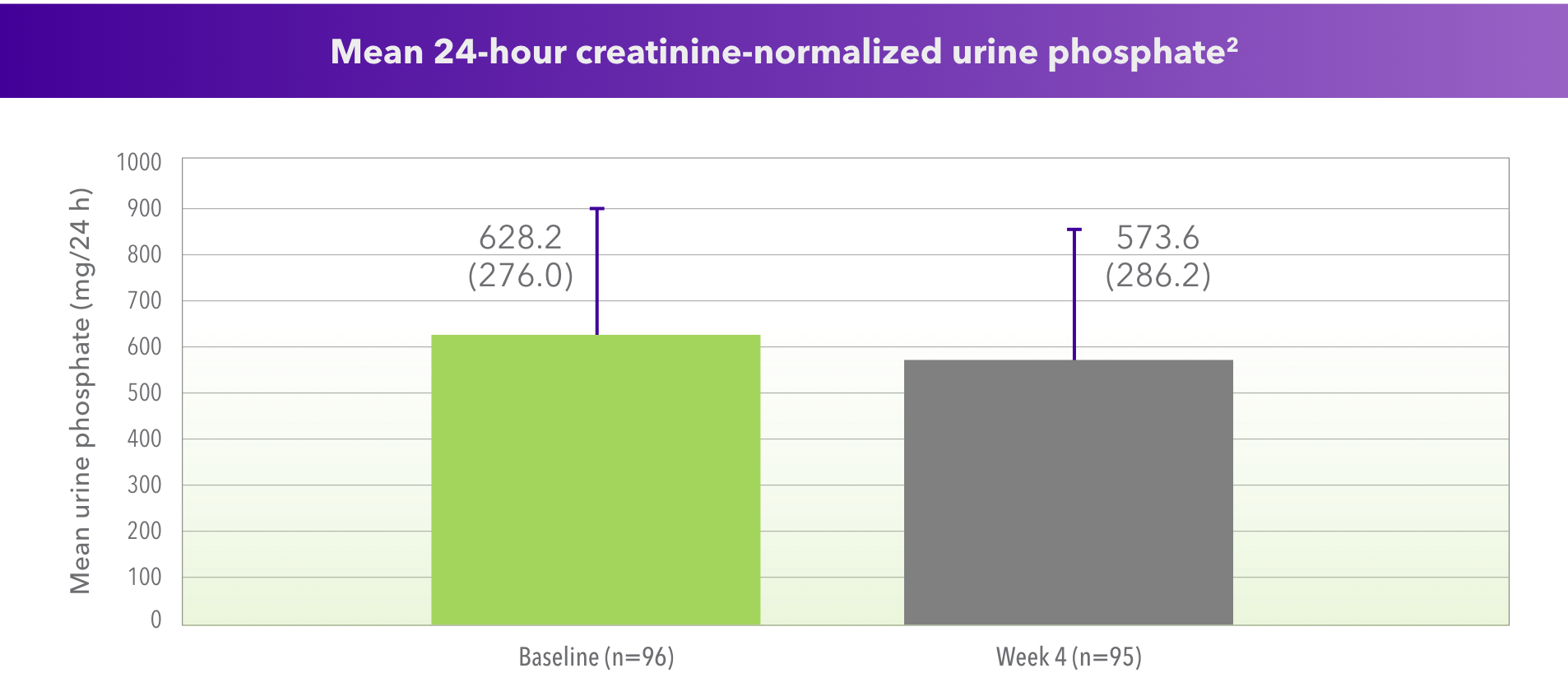
For Phosphate
- Median change: -43 mg/24 h (P=0.004, n=95)
- No patients had hypophosphatemia (< 2.5 mg/dL) during study period
*Baseline serum K+ = > 5.0 to 5.5 mEq/L.
†Baseline serum K+ = > 5.5 to < 6.0 mEq/L.
‡AMETHYST-DN 52-week study (N=304). Dosing studied, but not an approved dose.
VELTASSA has a well-tolerated GI profile
NextINDICATION
VELTASSA is indicated for the treatment of hyperkalemia in adults and pediatric patients ages 12 years and older.
Limitation of Use: VELTASSA should not be used as an emergency treatment for life-threatening hyperkalemia because of its delayed onset of action.
INDICATION & Important Safety Information
Important Safety Information
VELTASSA is indicated for the treatment of hyperkalemia.
Limitation of Use: VELTASSA should not be used as an emergency treatment for life-threatening hyperkalemia because of its delayed onset of action.
CONTRAINDICATIONS
VELTASSA is contraindicated in patients with a history of a hypersensitivity reaction to VELTASSA or any of its components.
INDICATION
VELTASSA is indicated for the treatment of hyperkalemia in adults and pediatric patients ages 12 years and older.
Limitation of Use: VELTASSA should not be used as an emergency treatment for life-threatening hyperkalemia because of its delayed onset of action.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
VELTASSA is contraindicated in patients with a history of a hypersensitivity reaction to VELTASSA or any of its components.
WARNINGS AND PRECAUTIONS
Worsening of Gastrointestinal Motility: Avoid use of VELTASSA in patients with severe constipation, bowel obstruction or impaction, including abnormal post-operative bowel motility disorders, because VELTASSA may be ineffective and may worsen gastrointestinal conditions. Patients with a history of bowel obstruction or major gastrointestinal surgery, severe gastrointestinal disorders, or swallowing disorders were not included in clinical studies.
Hypomagnesemia: VELTASSA binds to magnesium in the colon, which can lead to hypomagnesemia. In clinical studies, hypomagnesemia was reported as an adverse reaction in 5.3% of adult patients treated with VELTASSA. Approximately 9% of adult patients in clinical trials developed hypomagnesemia with a serum magnesium value < 1.4 mg/dL. Monitor serum magnesium. Consider magnesium supplementation in patients who develop low serum magnesium levels.
MOST COMMON ADVERSE REACTIONS
The most common adverse reactions (incidence ≥ 2%) in adult patients treated with VELTASSA were constipation (7.2%), hypomagnesemia (5.3%), diarrhea (4.8%), nausea (2.3%), abdominal discomfort (2.0%) and flatulence (2.0%). Mild to moderate hypersensitivity reactions were reported in 0.3% of adult patients treated with VELTASSA and included edema of the lips. The safety profile of VELTASSA in a study of 14 pediatric patients ages 12 to 17 years was generally similar to that observed in adult patients.