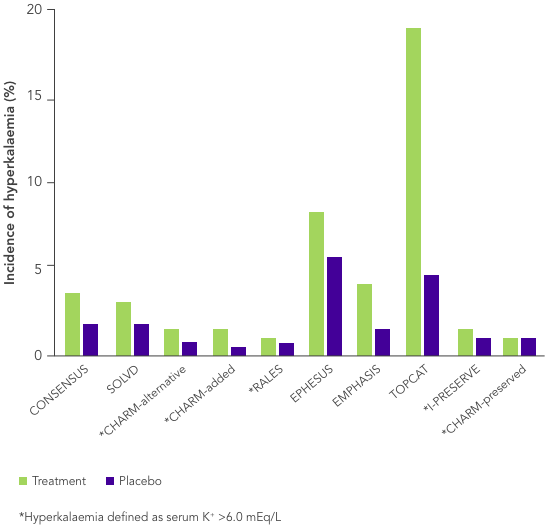
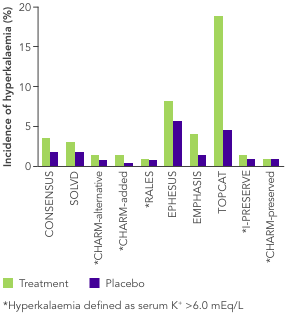
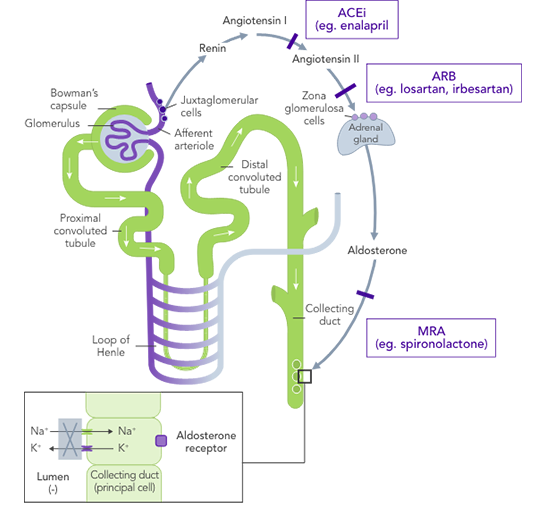
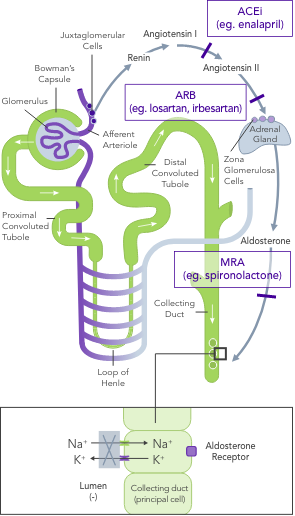
ACEi, angiotensin II converting enzyme inhibitor; ARB, angiotensin II-receptor blocker; ARNi, angiotensin-receptor neprilysin inhibitor; CHARM, Candesartan in Heart Failure-Assessment of Reduction in Mortality and Morbidity; CI, confidence interval; CONSENSUS, Cooperative North Scandinavian Enalapril Survival Study; CPS, calcium polystyrene sulfonate; CKD, chronic kidney disease; CV, cardiovascular; DM, diabetes mellitus; EMPHASIS, Eplerenone in Mild Patients Hospitalization and Survival Study; EPHESUS, Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HK, hyperkalaemia; HOPE, Heart Outcomes Prevention Evaluation; HR, hazard ratio; HTN, hypertension; I-PRESERVE, Irbesartan in HF patients with a preserved ejection fraction; K+, potassium ions; MI, myocardial infarction; MRA, mineralocorticoid-receptor antagonist; NYHA, New York Heart Association; PARADIGM, Prospective Comparison of ARNi with ACEi to Determine Impact on Global Mortality and Morbidity; PARAGON, Prospective Comparison of ARNi with ARBi Global Outcomes in HF with Preserved Ejection Fraction; QUALIFY, The Quality of Adherence to Guideline Recommendations for Life-saving Treatment in Heart Failure; RAASi, renin−angiotensin−aldosterone system inhibitors; RALES, Randomized Aldactone Evaluation Study; SOLVD, Studies of Left Ventricular Dysfunction; SPS, sodium polystyrene sulfonate; TOPCAT, Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist.
References:
1. Thomsen RW, et al. J Am Heart Assoc 2018;7(11):e008912. 2. Komajda M, et al. Eur J Heart Fail 2016;18(5):514−22. 3. Ponikowski P, et al. Eur J Heart Fail 2016;18(8):891−975. 4. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 2000;355(9200):253−9. 5. SOLVD Investigators. N Engl J Med 1991;325(5):293−302. 6. Desai AS, et al. J Am Coll Cardiol 2007;50(20):1959–66. 7. Pitt B, et al. N Engl J Med 1999;341(10):709−17. 8. Zannad F, et al. N Engl J Med 2011;364:11–21. 9. McMurray JJ, et al. N Engl J Med 2014;371(11):993−1004. 10. Solomon SD, et al. N Engl J Med 2019;381(17):1609−20. 11. Yancy CW, et al. Circulation 2017;136(6):e137−e161. 12. Palmer BF. N Engl J Med 2004;351(6):585−92. 13. Tromp T, van der Meer P. Eur Heart J 2019;21(Suppl A):A6−11. 14. Yancy CW, et al. J Am Coll Cardiol 2013;62(16):e147−e239. 15. Lindenfeld J, et al. J Card Fail 2010;16(6):e1−e194. 16. Trevisan M, et al. Eur J Heart Fail 2018;20(8):1217−26. 17. Epstein M, et al. Am J Manag Care 2015;21(11 Suppl):S212−S220. 18. Ouwerkerk W, et al. Eur Heart J 2017;38(24):1883−90. 19. Martens P, et al. Acta Cardiol 2020; doi: 10.1080/00015385.2020.1771885. 20. Dunn JD, et al. Am J Manag Care 2015;21(15 Suppl):S307−S315. 21. National Kidney Foundation. The DASH Diet. Available at: kidney.org/atoz/content/Dash_Diet (accessed July 2020). 22. Palmer SC, et al. Cochrane Database Syst Rev 2017;4:CD011998. 23. Vendramini LC, et al. Braz J Med Biol Res 2012;45(9):834−40. 24. Calcium Resonium PI. Sanofi 2018. 25. Kayexalate® US PI. Sanofi 2017. 26. Ter Maaten JM, et al. Clin Res Cardiol 2020;109(8):1048−59. 27. Kapelios CJ, et al. Eur J Heart Fail 2020; doi: 10.1002/ejhf.1796.