Report adverse events:
Adverse events should be reported to the Vifor Pharma group.
safety@viforpharma.com

I am practicing from:

Austria

Austria

Belgium

Germany

Ireland

Italy

Netherlands

Portugal

Spain

Switzerland
Other
I am residing in:

Austria

Austria

Belgium

Germany

Ireland

Italy

Netherlands

Portugal

Spain

Switzerland
Other

Bitte verwenden Sie als Benutzernamen die E-Mail Adresse, welche Sie bei der Registration angegeben hatten. Falls Sie das Passwort vergessen haben, können Sie sich einen Reaktivierungslink zustellen lassen. Alternativ ist für bereits registrierte Health Professionals auch eine Anmeldung mit der HPC-Card von FMH oder pharmaSuisse möglich.
Reliable potassium control around the clock, proven to keep your patients on guideline-recommended RAASi treatment1-11
Adverse events should be reported to the Vifor Pharma group.
safety@viforpharma.com
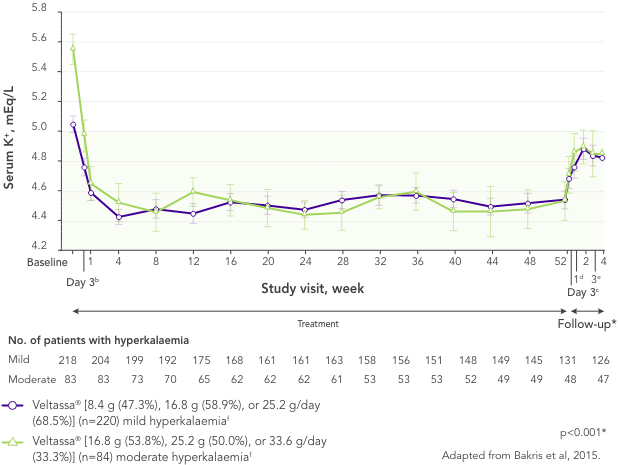

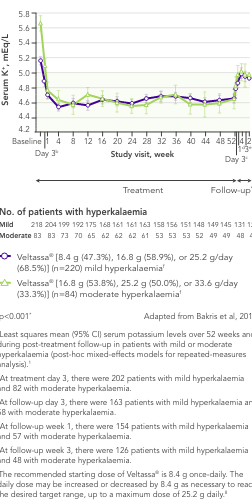
In 306 patients with T2DM, CKD, and HTN, treated with RAASi, significant decreases in serum K+ were observed after 4 weeks with Veltassa® and maintained for 1 year1 in:
![]()
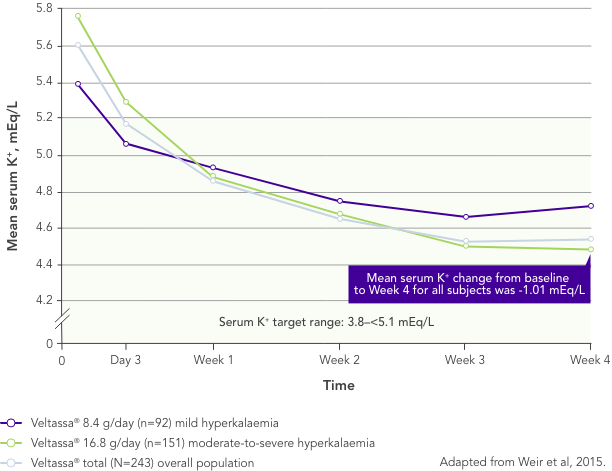

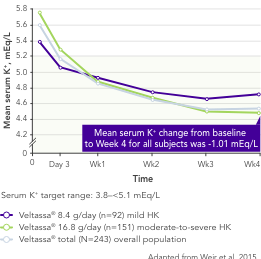
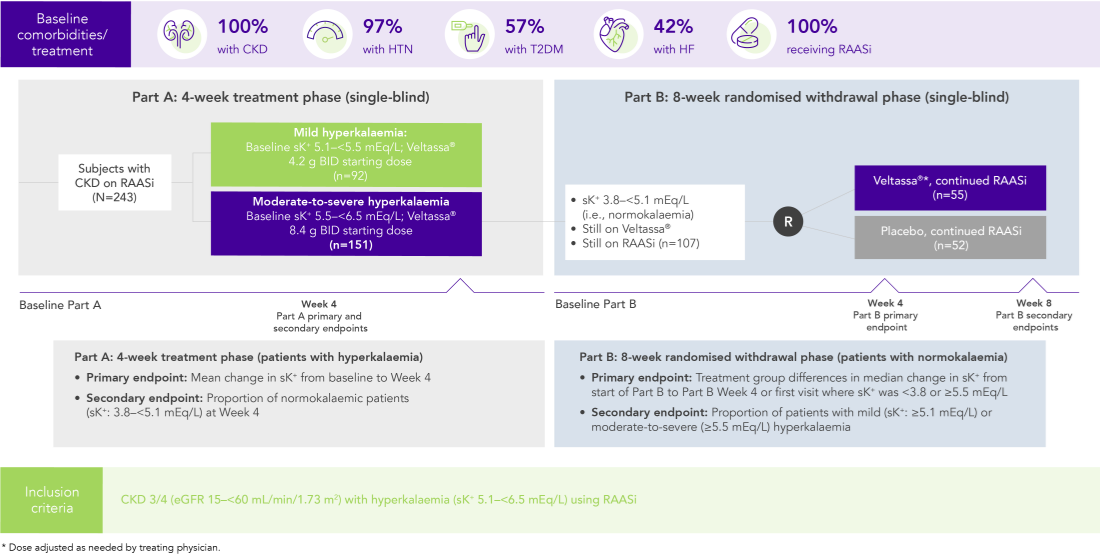
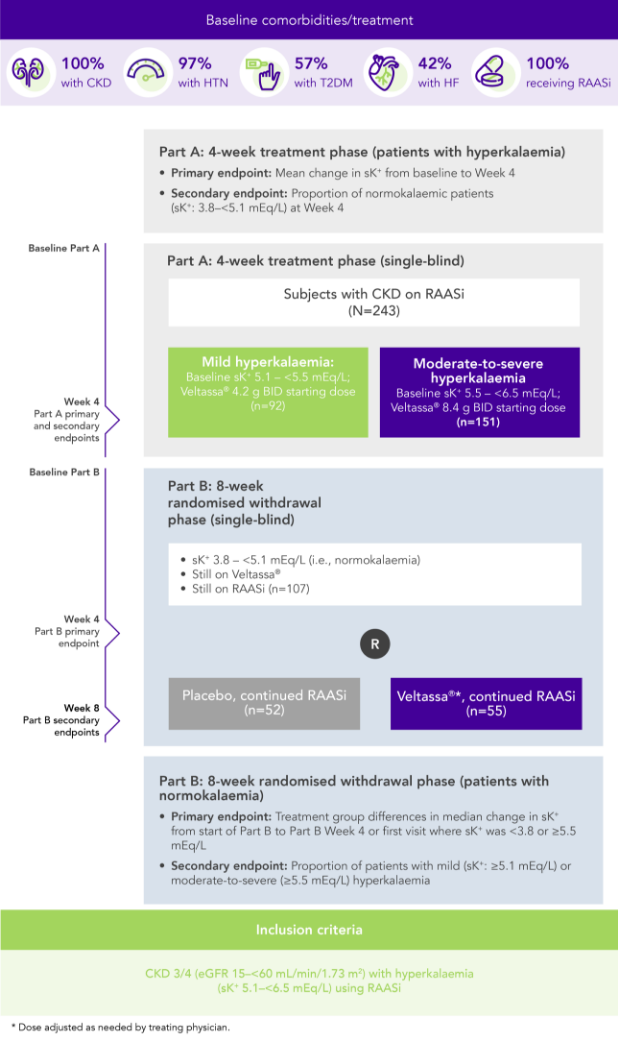
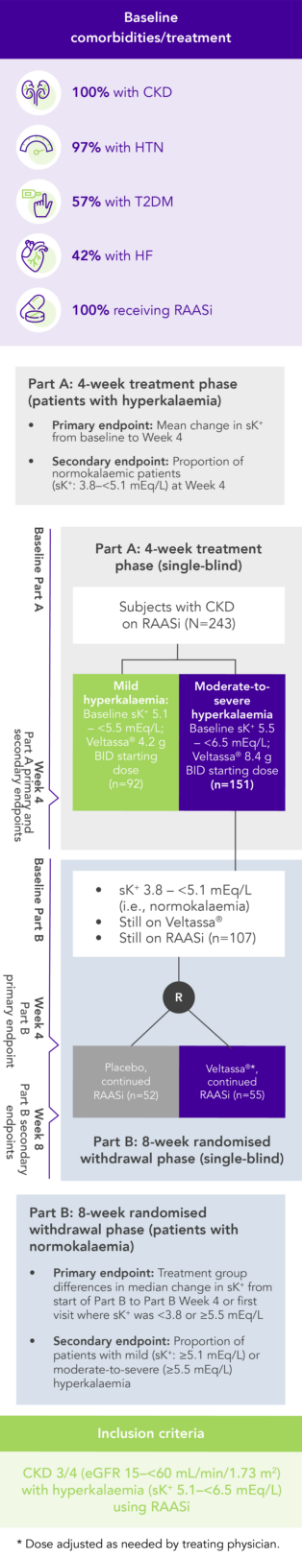
Among 243 patients with CKD and hyperkalaemia receiving RAASi, 76% had serum K+ in the target range (3.8–<5.1 mEq/L) at Week 42
Similar results in patients with:
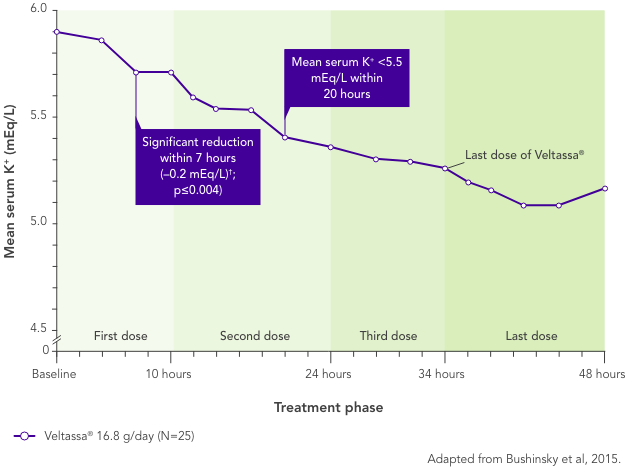

In patients with CKD and hyperkalaemia on RAASi, Veltassa® resulted in serum K+ reductions 4–7 hours after the first dose,3 with mean baseline serum K+ reduced at all subsequent times through 48 hours
Serum K+ did not increase before the next dose or for 24 hours after the last dose3
OPAL (N=243)2
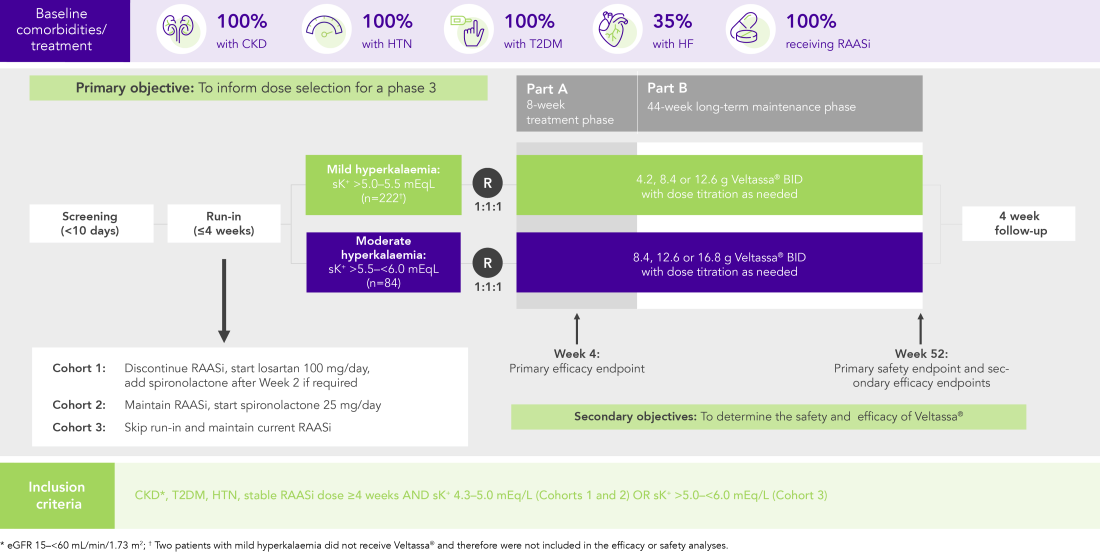
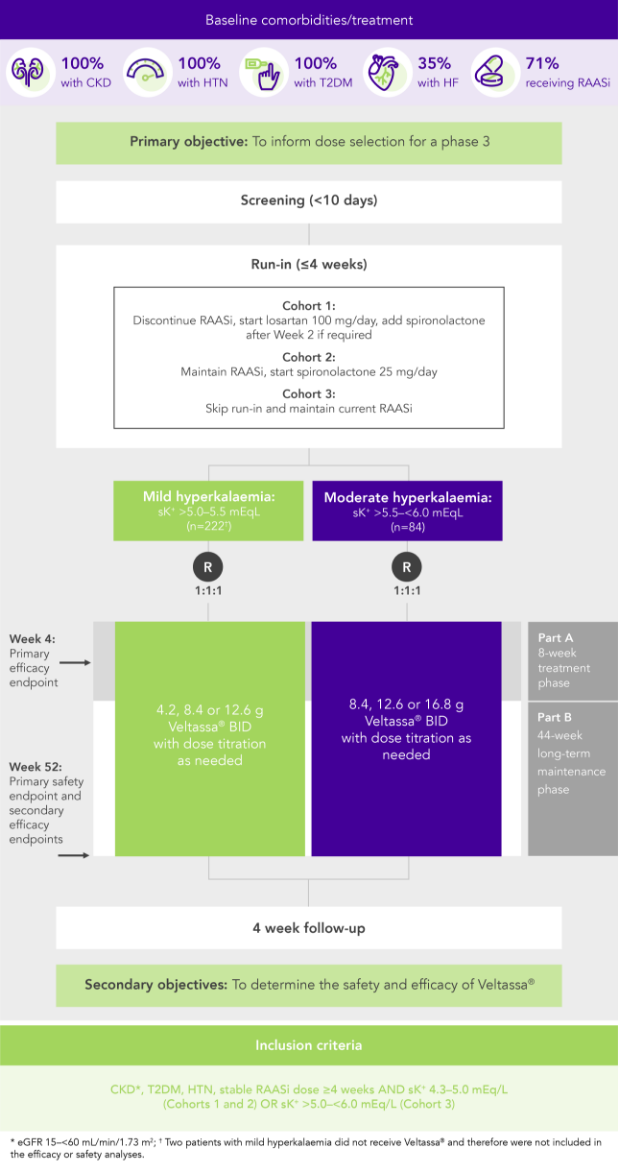
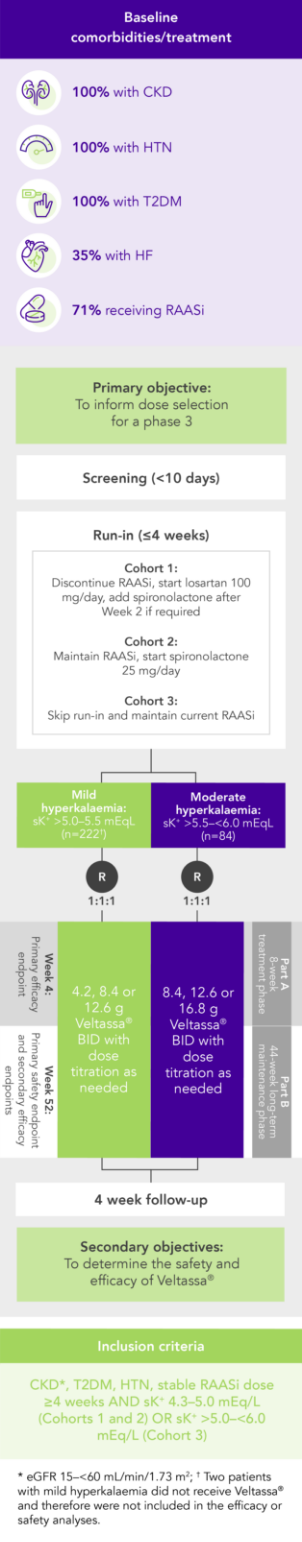
100% RAASi; 42% HF; 100% CKD; 97% HTN; 57% T2DM
Proportion of patients receiving any RAASi dose at Week 8 of the randomised withdrawal phase
(exploratory endpoint)
![]()
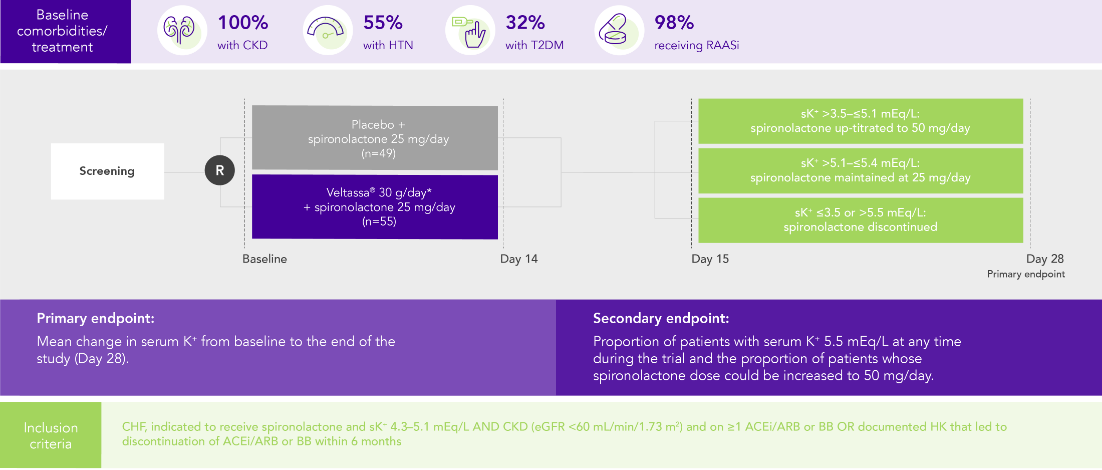
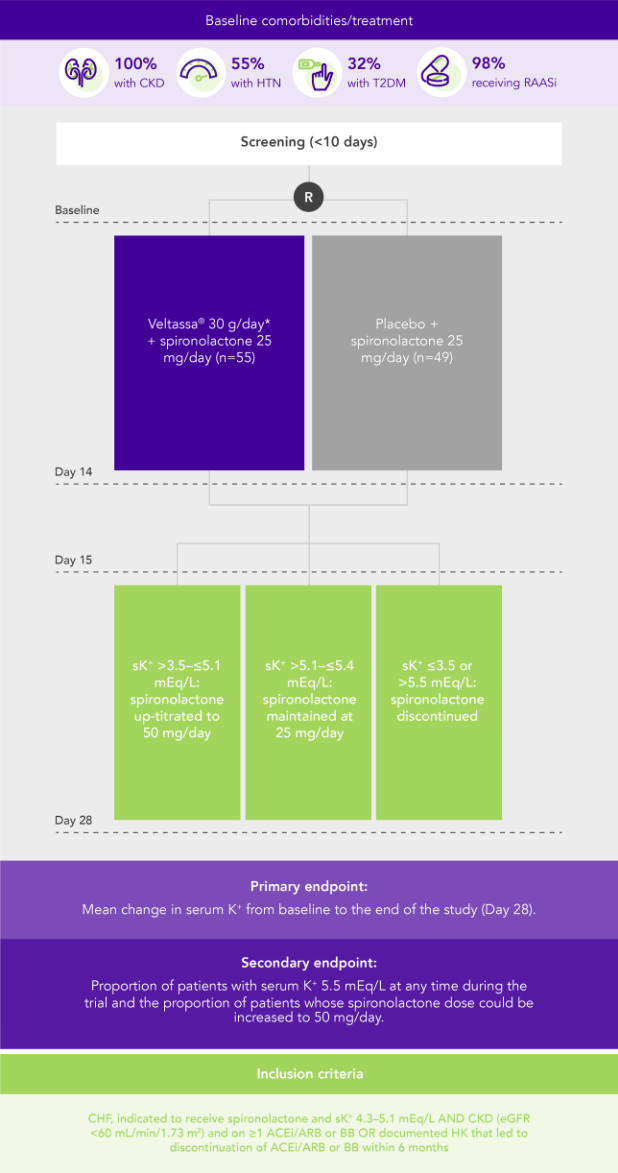
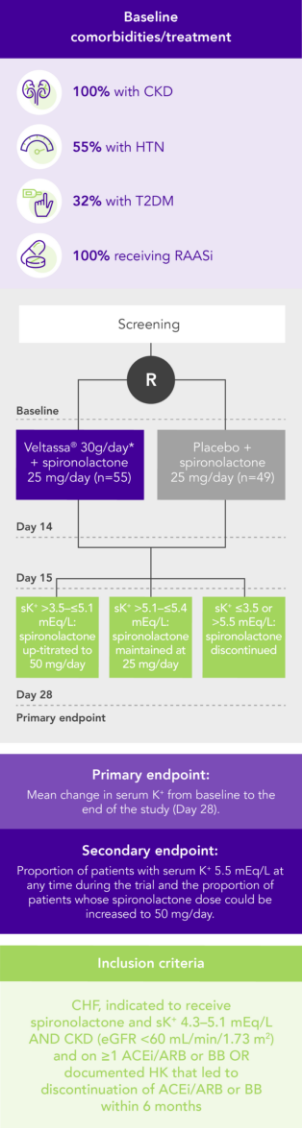
PEARL-HF (N=105)4
100% HF; 57% CKD; 32% T2DM; 98% RAASi
Proportion of patients up-titrated to spironolactone 50 mg/day
(secondary endpoint)
![]()
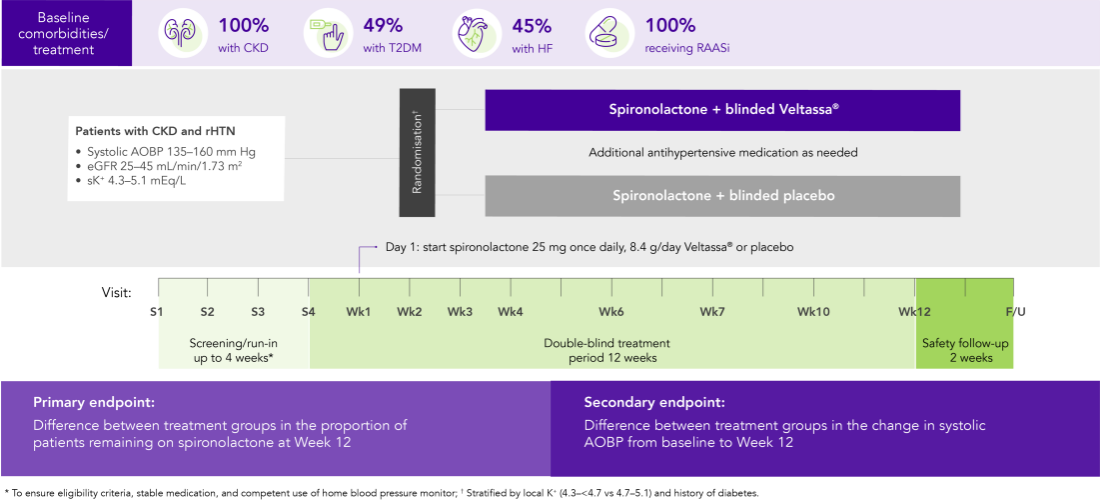
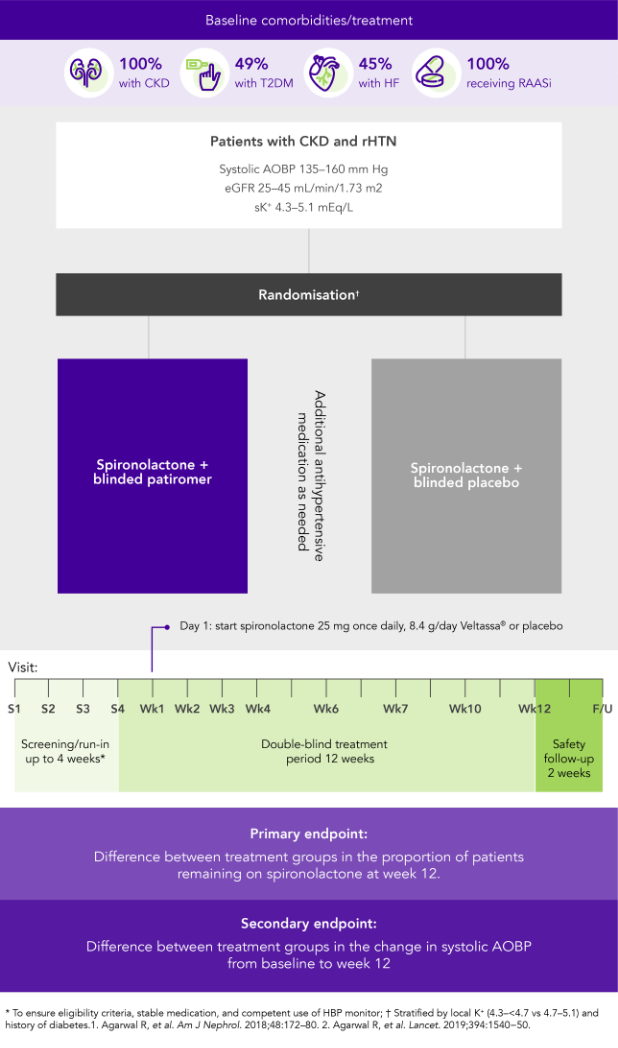
AMBER (N=295)5
100% CKD; 45% HF; 49% T2DM; 100% RAASi
Proportion of patients remaining on spironolactone at Week 12
(primary endpoint)
![]()
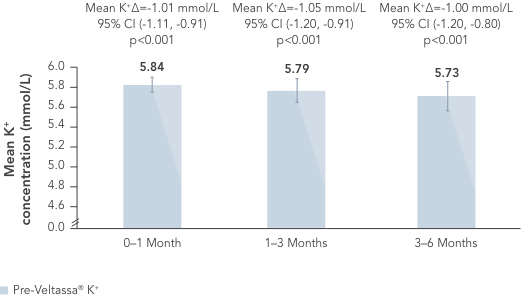
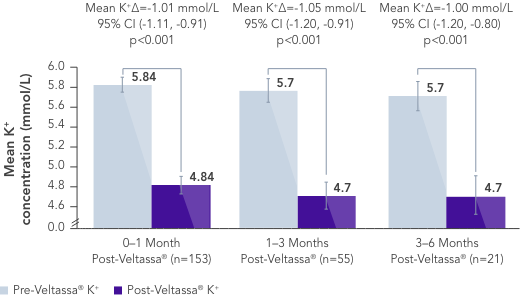
In a retrospective observational study with 288 hyperkalaemic patients with HF, T2DM or CKD, Veltassa® decreased serum K+ by an average of -1.0 mEq/L vs before treatment (p<0.001).6
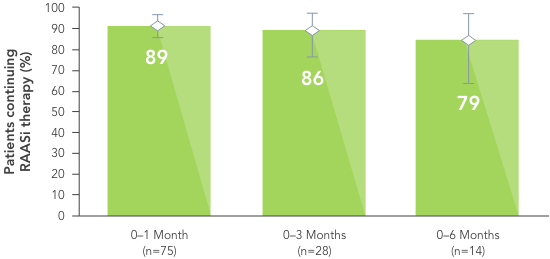
In the same study, RAASi therapy was continued in ~80% of patients for 6 months.6
Similarly, in a large US healthcare database including patients with hyperkalaemia, more patients receiving Veltassa® continued RAASi therapy vs those receiving a different serum K+ binder (SPS) or no serum K+ binder.7
Treatment with Veltassa® was also associated with fewer hospital admissions and emergency department visits.7








![]()
![]()
ADRs IN CLINICAL STUDIES
| System Organ Class | Common | Uncommon |
|---|---|---|
Metabolism and nutrition disorders |
Hypomagnesaemia: 5.3% |
|
Gastrointestinal disorders |
Constipation: 6.2% Diarrhoea: 3% Abdominal pain: 2.9% Flatulence: 1.8% |
Nausea Vomiting |
![]()
![]()
The favourable safety profile observed in clinical trials is supported by evidence from real-world studies.
Concomitant administration of Veltassa® showed reduced bioavailability of only 3 drugs:8
No interaction was found with administration of oral drugs at least 3 hours apart from Veltassa®. Quinidine showed a potential drug interaction in vitro. As a precautionary measure, administration of Veltassa® should therefore be separated by at least 3 hours from other oral medicinal products.8
![]()
LUNCH
3H >
![]()
SNACK
3H >
![]()
DINNER
VELTASSA® can be administered with or without food
At least 3 hours should be left between taking Veltassa® and any other medication
![]()
![]()
99.4%
receiving RAASi
at baseline
![]()
72.8%
had diabetes
![]()
81.2%
had CKD with eGFR
<60 mL/min/1.73 m2
![]()
48.7%
had HF

ACEi, angiotensin converting enzyme inhibitor; ADR, adverse drug reaction; AE, adverse event; AOBP, automated office blood pressure; ARB, angiotensin II receptor blocker; BB, beta blocker; BID, twice daily; Ca2+, calcium ions; CHF, congestive heart failure; CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; GI, gastrointestinal; HF, heart failure; HK, hyperkalaemia; HTN, hypertension; K+, potassium ions; mEqL, milliequivalents per litre; RAASi, renin‐angiotensin‐aldosterone system inhibitors; rHTN, resistant hypertension; sK+, serum potassium; SPS, sodium polystyrene sulfonate; T2DM, type 2 diabetes mellitus; US, United States
aLeast squares mean (95% CI) serum potassium levels over 52 weeks and during post-treatment follow-up in patients with mild or moderate hyperkalaemia (post-hoc mixed-effects models for repeated-measures analysis).1
bAt treatment day 3, there were 202 patients with mild hyperkalaemia and 82 with moderate hyperkalaemia.
cAt follow-up day 3, there were 163 patients with mild hyperkalaemia and 58 with moderate hyperkalaemia.
dAt follow-up week 1, there were 154 patients with mild hyperkalaemia and 57 with moderate hyperkalaemia.
eAt follow-up week 3, there were 126 patients with mild hyperkalaemia and 48 with moderate hyperkalaemia.
fThe recommended starting dose of Veltassa® is 8.4 g once-daily. The daily dose may be increased or decreased by 8.4 g as necessary to reach the desired target range, up to a maximum dose of 25.2 g daily.8
1. Bakris GL, et al. JAMA 2015;314(2):151−61. 2. Weir MR, et al. N Engl J Med 2015;372(3):211−21. 3. Bushinsky DA, et al. Kidney Int 2015;88(6):1427−33. 4. Pitt B, et al. Eur Heart J 2011;32(7):820−8. 5. Agarwal R, et al. Lancet 2019;394(10208):1540−50. 6. Kovesdy CP, et al. Postgrad Med 2020;132:176–83. 7. Desai NR, et al. PLoS One 2020 Jan 7;15(1):e0226844. doi: 10.1371/journal.pone.0226844. 8. Veltassa® SmPC. 9. Li L, et al. J Cardiovasc Pharmacol Ther 2016;21(5):456−65. 10. Data on file, Relypsa Inc. 11. Pitt B, et al. ESC Heart Fail 2018;5(4):592−602. 12. Seferovic PM, et al. Eur J Heart Fail 2019;21:1169–86. 13. KDIGO Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease Kidney Int 2020;98(Suppl 4S):S1–S116.