Report adverse events:
Adverse events should be reported to the Vifor Pharma group.
safety@viforpharma.com

I am practicing from:

Austria

Austria

Belgium

Germany

Ireland

Italy

Netherlands

Portugal

Spain

Switzerland
Other
I am residing in:

Austria

Austria

Belgium

Germany

Ireland

Italy

Netherlands

Portugal

Spain

Switzerland
Other

Bitte verwenden Sie als Benutzernamen die E-Mail Adresse, welche Sie bei der Registration angegeben hatten. Falls Sie das Passwort vergessen haben, können Sie sich einen Reaktivierungslink zustellen lassen. Alternativ ist für bereits registrierte Health Professionals auch eine Anmeldung mit der HPC-Card von FMH oder pharmaSuisse möglich.
Adverse events should be reported to the Vifor Pharma group.
safety@viforpharma.com
Providing sustained, around the clock control of your patients’ K+, and proven to enable guideline-recommended RAASi treatment, Veltassa® is reliable and easy to use with no sodium-associated risk1–7
![]()
![]()
![]()
![]()
![]()
![]()
![]()
MIX
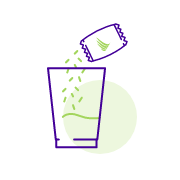
Mix 3 tablespoons of water with an entire sachet of Veltassa®, then stir
ADD

Add another 3 tablespoons of water and stir thoroughly. The powder will not dissolve and the mixture will look cloudy
DRINK

Drink immediately. If powder remains in the glass after drinking, repeat steps 2 and 3 to ensure the entire dose is taken
ADR, adverse drug reaction; Ca2+, calcium ions; CKD, chronic kidney disease; CV, cardiovascular; HK, hyperkalaemia; K+, potassium ions; MOA, mechanism of action; Na+, sodium ions; RAASi, renin-angiotensin-aldosterone system inhibitors.
Veltassa® is indicated for the treatment of hyperkalaemia in adults.7
1. Weir MR, et al. N Engl J Med 2015;372(3):211–21. 2. Bakris GL, et al. JAMA 2015;314(2):151–61. 3. Bushinsky DA, et al. Kidney Int 2015;88:1427–33. 4. Pitt B, et al. Eur Heart J 2011;32:820–8. 5. Agarwal R, et al. Lancet 2019;394(10208):1540−50. 6. Li L, et al. J Card Pharmacol Ther 2016;21(5):456–65. 7. Veltassa® EU SmPC, 2019. 8. Di Lullo L, et al. Cardiorenal Med 2019;9(1):8−21. 9. Rastegar A, Soleimani M. Postgrad Med J 2001;77(914):759−64. 10. Kjeldsen KP, Schmidt TA. Eur Heart J 2019;21(Suppl A):A2−A5. 11. Collins AJ, et al. Am J Nephrol 2017;46(3):213−21. 12. Kovesdy CP, et al. Eur Heart J 2018;39(17):1535−42. 13. Rossignol P, et al. Clin Kidney J 2020;13:714−9. 14. Palmer BF, et al. N Engl J Med 2004;351:585–92. 15. KDIGO Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int 2020;98(Suppl 4S):S1–S116. 16. KDIGO Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013;3:1–150. 17. National Kidney Foundation. K/DOQI Clinical Practice Guidelines on Hypertension and Antihypertensive Agents in Chronic Kidney Disease. 2004. Available at: kidneyfoundation.cachefly.net/professionals/KDOQI/guidelines_bp/index.htm (accessed July 2020). 18. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 2000;355(9200):253−9. 19. SOLVD Investigators. N Engl J Med 1991;325(5):293−302. 20. Desai AS, et al. J Am Coll Cardiol 2007;50(20):1959−66. 21. Pitt B, et al. N Engl J Med 1999;341(10):709−17. 22. Zannad F, et al. N Engl J Med 2011;364:11–21. 23. McMurray JJ, et al. N Engl J Med 2014;371(11):993−1004. 24. Solomon SD, et al. N Engl J Med 2019;381(17):1609−20. 25. Epstein M, et al. Am J Manag Care 2015;21(11 Suppl):S212−S220. 26. Kovesdy CP, et al. Postgrad Med 2020;132:176–83. 27. Data on file. Healthcare Analytics (SHA), a Symphony Health Solutions Corporation (February 2019).