Report adverse events:
Adverse events should be reported to the Vifor Pharma group.
safety@viforpharma.com

I am practicing from:

Austria

Austria

Belgium

Germany

Ireland

Italy

Netherlands

Portugal

Spain

Switzerland
Other
I am residing in:

Austria

Austria

Belgium

Germany

Ireland

Italy

Netherlands

Portugal

Spain

Switzerland
Other

Bitte verwenden Sie als Benutzernamen die E-Mail Adresse, welche Sie bei der Registration angegeben hatten. Falls Sie das Passwort vergessen haben, können Sie sich einen Reaktivierungslink zustellen lassen. Alternativ ist für bereits registrierte Health Professionals auch eine Anmeldung mit der HPC-Card von FMH oder pharmaSuisse möglich.
Adverse events should be reported to the Vifor Pharma group.
safety@viforpharma.com
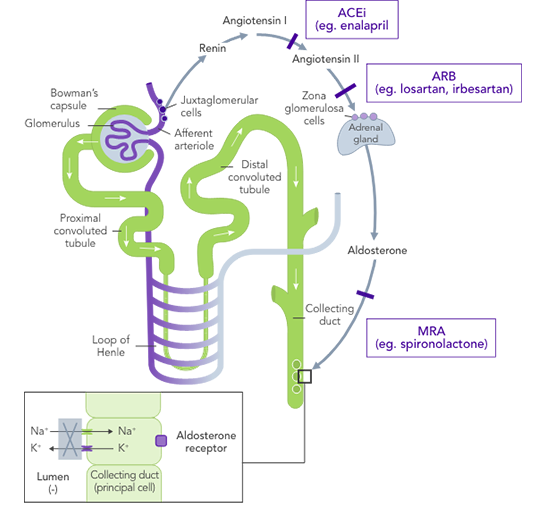
ACEis
HOPE (N=3,577)4
SOLVD (N=2,569)5
Reduction in combined outcome of MI, stroke or CV mortality in patients at high CV risk4
Risk reduction of HF-related hospital admissions or mortality in patients with congestive HF5
ARBs
CHARM (N=7,599)6
Risk reduction in CV mortality or HF hospitalisation in patients with HF6
MRAs
RALES (N=1,663)7
EMPHASIS (N=2,737)8
Reduction in risk of mortality in patients with HFrEF7
Improvement in NYHA class7
Lower frequency of hospitalisation for worsening HFrEF7
Risk reduction in CV mortality or hospitalisation due to HFrEF8
ARNis
PARADIGM-HF (N=8,442)9
PARAGON-HF (N=4,822)10
Reduction in risk of hospitalisation in patients with HFrEF9
Reduction in risk of CV mortality in patients with HFrEF9
Reduction in risk of HF hospitalisations in patients with HFpEF10
HR 0.85; 95% CI, 0.72–1.00
Reduction in risk of CV mortality in patients with HFpEF10
HR 0.95; 95% CI, 0.79–1.16






7,092
Outpatients
63.1
Mean age
82%
NYHA class II/III
Baseline
comorbidities
![]()
64.6%
With HTN
![]()
34.3%
With DM
![]()
17.8%
With CKD
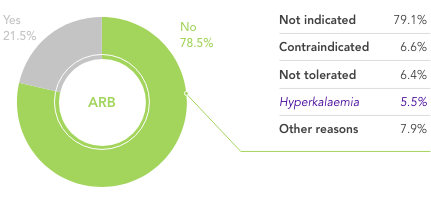
Study information
QUALIFY was an international prospective longitudinal survey of outpatients with chronic HF recruited 1–15 months after hospitalisation for HF
![]()
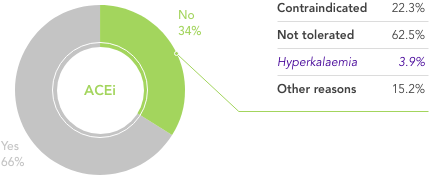
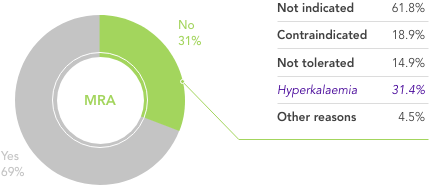
What is needed is a therapy that allows HF patients to stay on beneficial RAASi therapy with reliable and predictable long-term control of serum K+ levels.
Dietary modifications may lead to restricted consumption of healthy foods,21 and it may be very difficult for patients already on a restricted diet to adhere to further dietary modifications.22,23 The effects of dietary interventions on outcomes are uncertain22
Traditional K+-binders (SPS and CPS) can be associated with life-threatening GI side effects and hypokalaemia24,25 and may not be suitable in those who cannot tolerate an increase in sodium (SPS)25
Loop diuretics at the lowest achievable dose are recommended for the symptomatic management of congestion in patients with HFrEF26
Their use in patients to manage hyperkalaemia can result in worsening of renal function, contraction of plasma volume, and lower blood pressure26
Their use may also hamper the up-titration of guideline-recommended doses of ACEi/ARB26
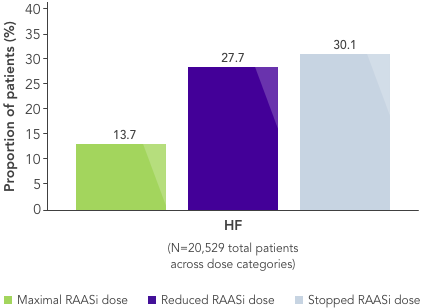
In a real-world observational study reduction in dose of loop diuretics was associated with better clinical outcomes vs no dose reduction, but 76% of patients remained on the same dose, contrary to guidelines27

There is an unmet need for a reliable long-term option for the management of chronic hyperkalaemia that enables patients to continue RAASi therapy at target doses20
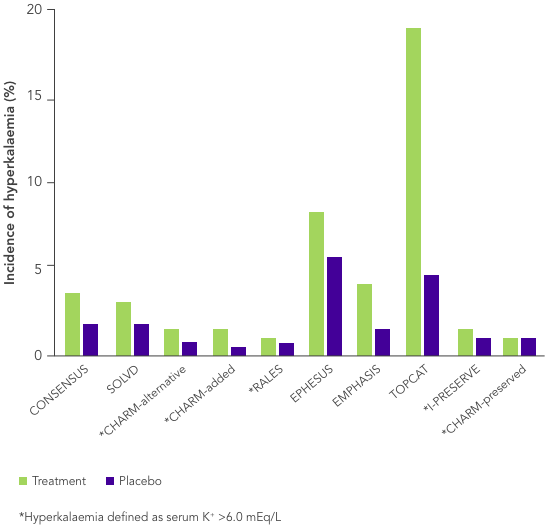
ACEi, angiotensin II converting enzyme inhibitor; ARB, angiotensin II-receptor blocker; ARNi, angiotensin-receptor neprilysin inhibitor; CHARM, Candesartan in Heart Failure-Assessment of Reduction in Mortality and Morbidity; CI, confidence interval; CONSENSUS, Cooperative North Scandinavian Enalapril Survival Study; CPS, calcium polystyrene sulfonate; CKD, chronic kidney disease; CV, cardiovascular; DM, diabetes mellitus; EMPHASIS, Eplerenone in Mild Patients Hospitalization and Survival Study; EPHESUS, Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HK, hyperkalaemia; HOPE, Heart Outcomes Prevention Evaluation; HR, hazard ratio; HTN, hypertension; I-PRESERVE, Irbesartan in HF patients with a preserved ejection fraction; K+, potassium ions; MI, myocardial infarction; MRA, mineralocorticoid-receptor antagonist; NYHA, New York Heart Association; PARADIGM, Prospective Comparison of ARNi with ACEi to Determine Impact on Global Mortality and Morbidity; PARAGON, Prospective Comparison of ARNi with ARBi Global Outcomes in HF with Preserved Ejection Fraction; QUALIFY, The Quality of Adherence to Guideline Recommendations for Life-saving Treatment in Heart Failure; RAASi, renin−angiotensin−aldosterone system inhibitors; RALES, Randomized Aldactone Evaluation Study; SOLVD, Studies of Left Ventricular Dysfunction; SPS, sodium polystyrene sulfonate; TOPCAT, Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist.
1. Thomsen RW, et al. J Am Heart Assoc 2018;7(11):e008912. 2. Komajda M, et al. Eur J Heart Fail 2016;18(5):514−22. 3. Ponikowski P, et al. Eur J Heart Fail 2016;18(8):891−975. 4. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 2000;355(9200):253−9. 5. SOLVD Investigators. N Engl J Med 1991;325(5):293−302. 6. Desai AS, et al. J Am Coll Cardiol 2007;50(20):1959–66. 7. Pitt B, et al. N Engl J Med 1999;341(10):709−17. 8. Zannad F, et al. N Engl J Med 2011;364:11–21. 9. McMurray JJ, et al. N Engl J Med 2014;371(11):993−1004. 10. Solomon SD, et al. N Engl J Med 2019;381(17):1609−20. 11. Yancy CW, et al. Circulation 2017;136(6):e137−e161. 12. Palmer BF. N Engl J Med 2004;351(6):585−92. 13. Tromp T, van der Meer P. Eur Heart J 2019;21(Suppl A):A6−11. 14. Yancy CW, et al. J Am Coll Cardiol 2013;62(16):e147−e239. 15. Lindenfeld J, et al. J Card Fail 2010;16(6):e1−e194. 16. Trevisan M, et al. Eur J Heart Fail 2018;20(8):1217−26. 17. Epstein M, et al. Am J Manag Care 2015;21(11 Suppl):S212−S220. 18. Ouwerkerk W, et al. Eur Heart J 2017;38(24):1883−90. 19. Martens P, et al. Acta Cardiol 2020; doi: 10.1080/00015385.2020.1771885. 20. Dunn JD, et al. Am J Manag Care 2015;21(15 Suppl):S307−S315. 21. National Kidney Foundation. The DASH Diet. Available at: kidney.org/atoz/content/Dash_Diet (accessed July 2020). 22. Palmer SC, et al. Cochrane Database Syst Rev 2017;4:CD011998. 23. Vendramini LC, et al. Braz J Med Biol Res 2012;45(9):834−40. 24. Calcium Resonium PI. Sanofi 2018. 25. Kayexalate® US PI. Sanofi 2017. 26. Ter Maaten JM, et al. Clin Res Cardiol 2020;109(8):1048−59. 27. Kapelios CJ, et al. Eur J Heart Fail 2020; doi: 10.1002/ejhf.1796.