Report adverse events:
Adverse events should be reported to the Vifor Pharma group.
safety@viforpharma.com

I am practicing from:

Austria

Austria

Belgium

Germany

Ireland

Italy

Netherlands

Portugal

Spain

Switzerland
Other
I am residing in:

Austria

Austria

Belgium

Germany

Ireland

Italy

Netherlands

Portugal

Spain

Switzerland
Other

Bitte verwenden Sie als Benutzernamen die E-Mail Adresse, welche Sie bei der Registration angegeben hatten. Falls Sie das Passwort vergessen haben, können Sie sich einen Reaktivierungslink zustellen lassen. Alternativ ist für bereits registrierte Health Professionals auch eine Anmeldung mit der HPC-Card von FMH oder pharmaSuisse möglich.
Adverse events should be reported to the Vifor Pharma group.
safety@viforpharma.com
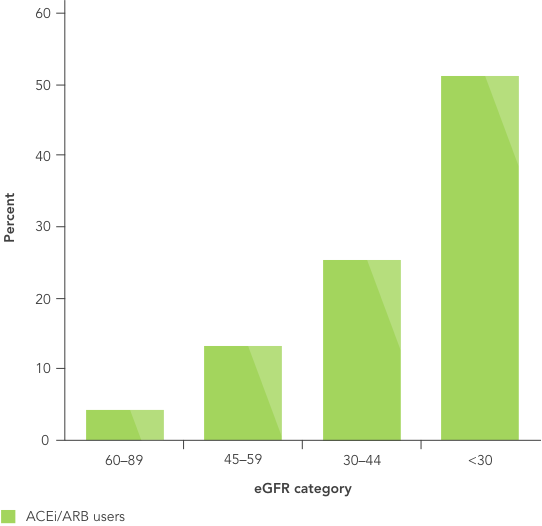
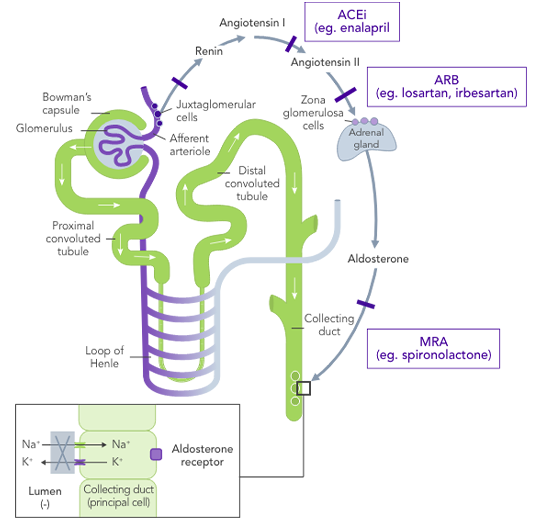
RAASi are the cornerstone of therapy in CKD, and their use at the highest tolerated dose is recommended by multiple organisations4,7
Patient population:
Patients with diabetic nephropathy
Treatment:
Once daily irbesartan 300 mg, amlodipine 10 mg or placebo
Primary endpoint:
Composite of death, ESRD or doubling of
baseline serum creatinine
Mean follow-up:
years
Risk reduction in primary endpoint:
vs placebo
(p=0.02)
vs amlodipine
(p=0.006)
Patient population:
Patients with diabetic nephropathy
Treatment:
Once daily losartan 50−100 mg or placebo
Primary endpoint:
Composite of death, ESRD or doubling of
baseline serum creatinine
Mean follow-up:
years
Risk reduction in primary endpoint:
vs placebo
(p=0.02)














![]()
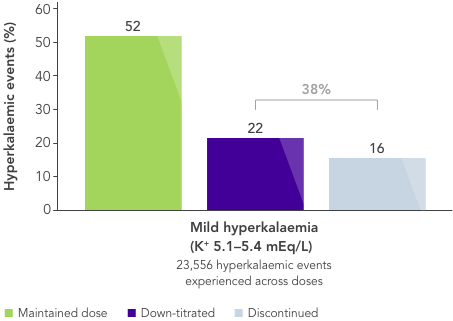
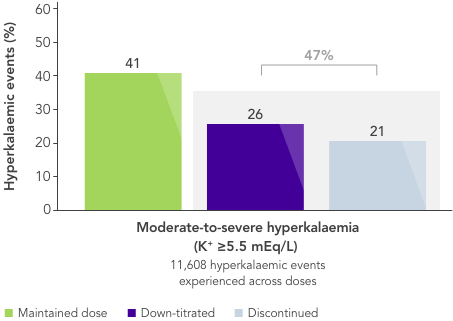
What is needed is a therapy that allows CKD patients to stay on beneficial RAASi therapy with reliable long-term control of serum K+ levels.
Dietary modifications may lead to restricted consumption of healthy foods,18 and it may be very difficult for patients already on a restricted diet to adhere to further dietary modifications.19,20 The effects of dietary interventions on outcomes are uncertain.19
Non-K+-sparing loop diuretics depend on residual renal function and are associated with increased risk of gout and diabetes, volume contraction and reduced potassium excretion, and sarcopenia.17,21
Traditional K+-binders (SPS and CPS) can be associated with life-threatening GI side effects and hypokalaemia22,23 and may not be suitable in those who cannot tolerate an increase in sodium (SPS).23

There is an unmet need for a reliable long-term option for the management of chronic hyperkalaemia that enables patients to continue RAASi therapy at target doses.17
ACEi, angiotensin II converting enzyme inhibitor; ARB, angiotensin II-receptor blocker; CKD, chronic kidney disease; CPS, calcium polystyrene sulfonate; ESRD, end-stage renal disease; eGFR, estimated glomerular filtration rate; GI, gastrointestinal; HK, hyperkalaemia; IDNT, Irbesartan Diabetic Nephropathy Trial; K+, potassium ions; MRA, mineralocorticoid-receptor antagonist; Na+, sodium ions; NIDDM, non-insulin-dependent diabetes mellitus; RENAAL, Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan; RAASi, renin−angiotensin−aldosterone system inhibitors; RR, relative risk; SPS, sodium polystyrene sulfonate; T2DM, type 2 diabetes mellitus; US, United States.
1. Rosano GMC, et al. Eur Heart J Cardiovasc Pharmacother 2018;4(3):180−8. 2. Epstein M, et al. Am J Manag Care 2015;21(11 Suppl):S212−S220. 3. Yildirim T, et al. Ren Fail 2012;34(9):1095−9. 4. KDIGO Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int 2020;98(Suppl 4S):S1–S116. 5. KDIGO Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013;3:1–150. 6. National Kidney Foundation. K/DOQI Clinical Practice Guidelines on Hypertension and Antihypertensive Agents in Chronic Kidney Disease. 2004. Available at: kidneyfoundation.cachefly.net/professionals/KDOQI/guidelines_bp/index.htm (accessed July 2020). 7. National Institute for Health and Care Excellence. Chronic kidney disease in adults: assessment and management. 2014. Available at: http://www.nice.org.uk/CG182 (accessed April 2020). 8. Lewis EJ, et al. N Engl J Med 2001;345(12):851−60. 9. Brenner BM, et al. N Engl J Med 2001;345(12):861−9. 10. The GISEN Group. Lancet 1997;28;349(9069):1857−63. 11. Palmer BF. N Engl J Med 2004;351(6):585−92. 12. Georgianos PI, Agarwal R. Kidney Int 2018;93(2):325−34. 13. Nilsson E, et al. Int J Cardiol 2017;245:277−84. 14. Einhorn LM, et al. Arch Intern Med 2009;169(12):1156−62. 15. Bandak G, et al. J Am Heart Assoc 2017;6(7):e005428. 16. Qiao Y, et al. JAMA Intern Med 2020;180(5):718−26. 17. Dunn JD, et al. Am J Manag Care. 2015;21(15 Suppl):S307−S315. 18. National Kidney Foundation. The DASH Diet. Available at: kidney.org/atoz/content/Dash_Diet (accessed July 2020). 19. Palmer SC, et al. Cochrane Database Syst Rev 2017;4:CD011998. 20. Vendramini LC, et al. Braz J Med Biol Res 2012;45(9):834−40. 21. Ishikawa S, et al. PLOS One 2018;13:1–16. 22. Calcium Resonium PI. Sanofi 2018. 23. Kayexalate® US PI. Sanofi 2017.